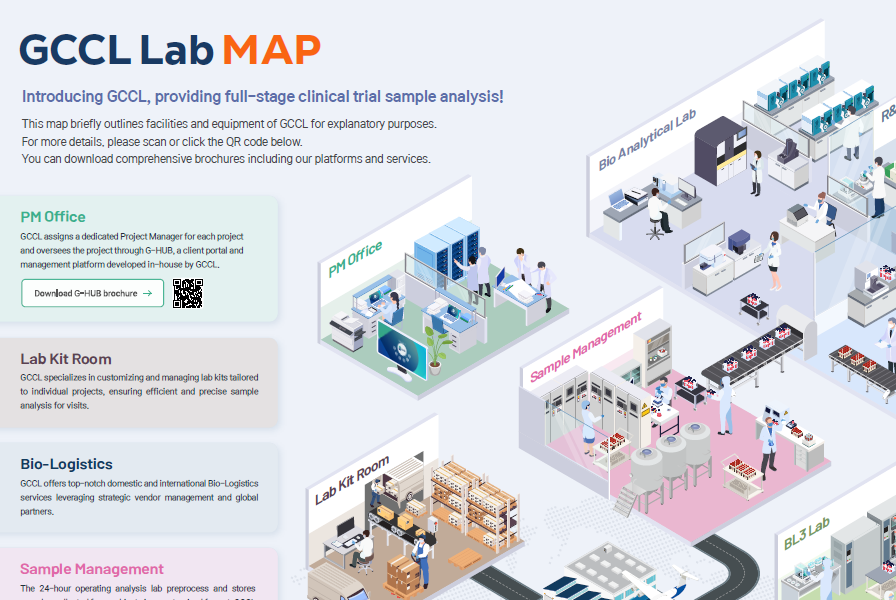
What we do
GCCL provides the best clinical trial sample analysis beyond simple central lab services.
Clinical Consulting
- PK analysis consulting
- Method development and validation
Central Laboratory
- Over 5,000 safety testing items (GCLP certified)
- Hematology, immunology, general biochemistry, microbiology, pathology, special biochemistry, genome analysis
Bio-Analytical
- PK analysis (GCLP certified)
- Biomarker analysis
- PD analysis through ELISpot, FACS, LC-MS/MS
Data Management
- Establishment of a computer system for each project
- Web page-based result reporting
- EDC upload through the validated system
Sample Processing
- PBMC isolation
- Sample preparation service according to the necessary requirements and study protocol
Clinical Collection Kits
- Production of customized Lab kits for each study and visit
- An exclusive Lab kit production room
Bio Logistics
- Transport using our own vehicles through over 50 directly managed domestic branches
- Use of exclusive transport boxes for clinical trials
- Overseas shipment through global partners
Biorepository
- Sample storage that meets qualifications such as controlled ambient, refrigeration, or frozen(-20℃, -70℃, -150℃)
- A real-time temperature monitoring and alarm system
- Security system

![[GCCL] Brochure](/board_data/0000000276/00000002762026000001_file1.png)